Jill
No I did not receive the listserv email.
Thank you,
Kevin ᷀.::. ꜘ
From: Jill M. Mortali [mailto:[log in to unmask]]
Sent: Monday, December 18, 2017 11:23 AM
To: [log in to unmask]
Subject: FW: Reminder NIH FORMS E - Important Changes to NIH Forms for Investigators
Importance: High
A few updates:
OSP Informational Session for NIH Researchers on Forms E:
Wednesday, January 10, 2018
4:00 PM – 4:45PM
Auditorium B, DHMC
The presentation will cover the new NIH Human Subjects and Clinical Trials Information Form by showing the additional required questions and how to select the correct FOA.
More sessions to be scheduled based upon demand.
RAPPORT Notes:
The ability to start NIH Forms E funding proposals in RAPPORT Grants will be effective on January 5. Please do not attempt to select one of the new NIH PAs or FOAs until January 5. Presently, OSP and a number of department grant
managers are testing the modifications to RAPPORT Grants to allow the changes coming, with Forms E, to function with a clear and easy process in the Funding Proposal Workspace of an application. Testing will continue through this week. If you have any question,
please email [log in to unmask]
FORMS E
Important Changes to NIH Forms
NIH will require the use of application packages with a Competition ID of ‘FORMS-E’ for due dates on or after
January 25, 2018.
Applications prepared using the prior ‘FORMS-D’ packages for due dates after
January 24, 2018 will be rejected without review by NIH.
Changes are focused on human subjects questions and clinical trials but other changes apply to all applications:

-
If your research involves human subjects in any way,
including human specimens and data, please be aware of the new questions below:
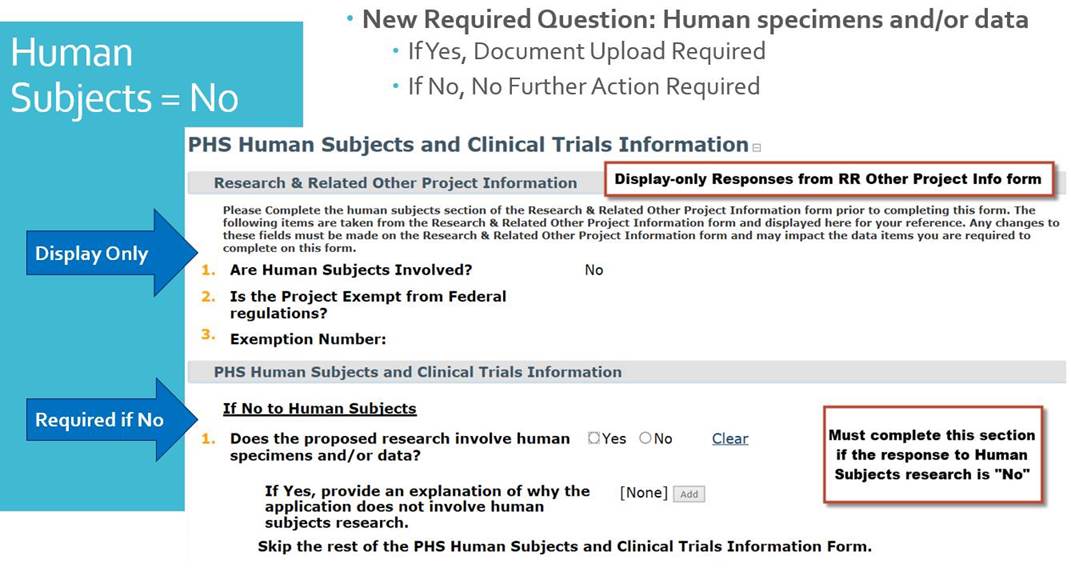
-
If “yes” to Human Subjects you will need to create a Study Record:

*Study Record Screen Shots Appear After the Resource Links Below*
Links to resources:
Is my project a clinical trial?
Decision-making tool:
https://grants.nih.gov/policy/clinical-trials/definition.htm
See example case studies here:
https://grants.nih.gov/policy/clinical-trials/case-studies.htm
FAQ:
https://grants.nih.gov/grants/policy/faq_clinical_trial_definition.htm
Training Video for the Study Record:
https://grants.nih.gov/policy/clinical-trials/new-human-subject-clinical-trial-info-form.htm
Instructions the Study Record:
https://grants.nih.gov/grants/how-to-apply-application-guide/forms-e/general/g.500-phs-human-subjects-and-clinical-trials-information.htm
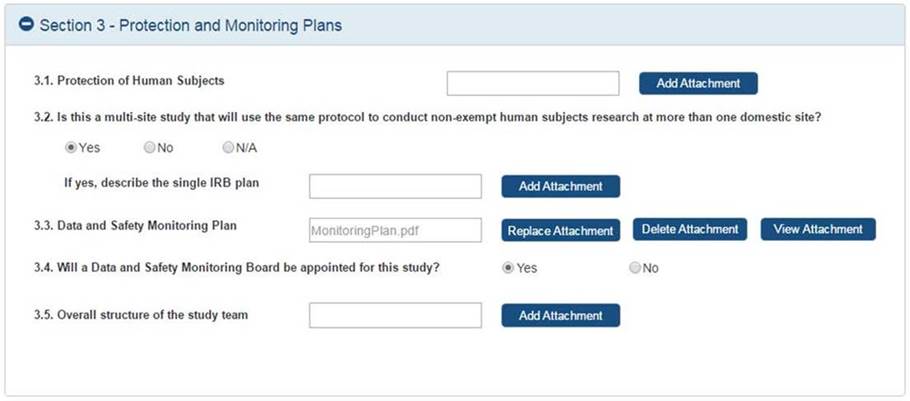
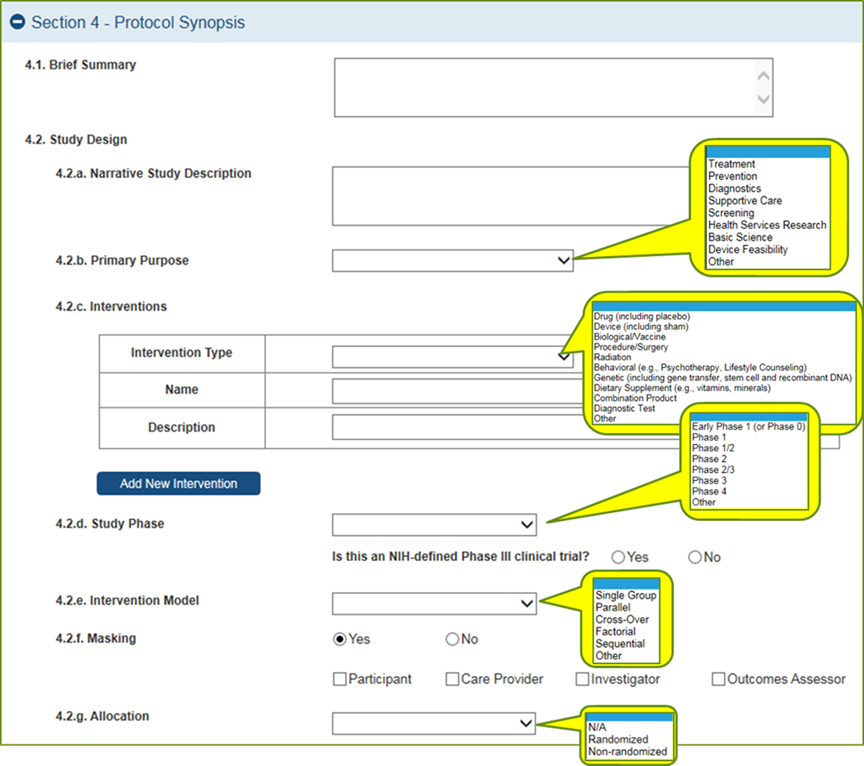
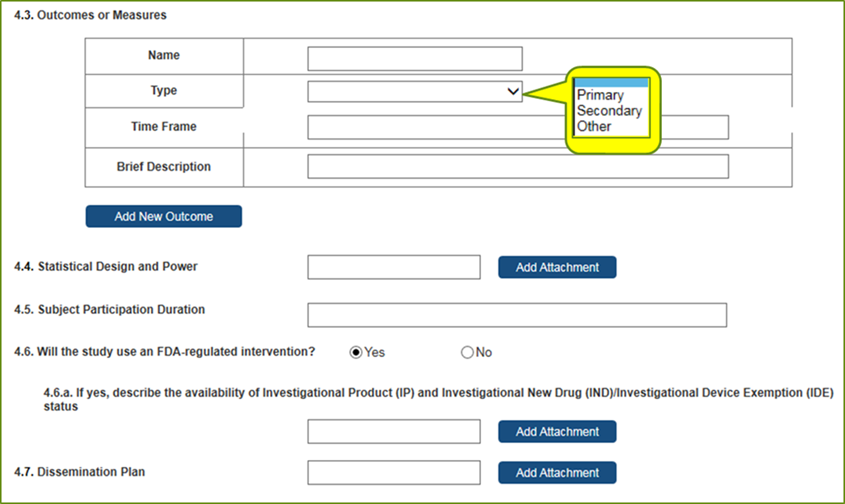
Screen Shots of New Form and Study Record:
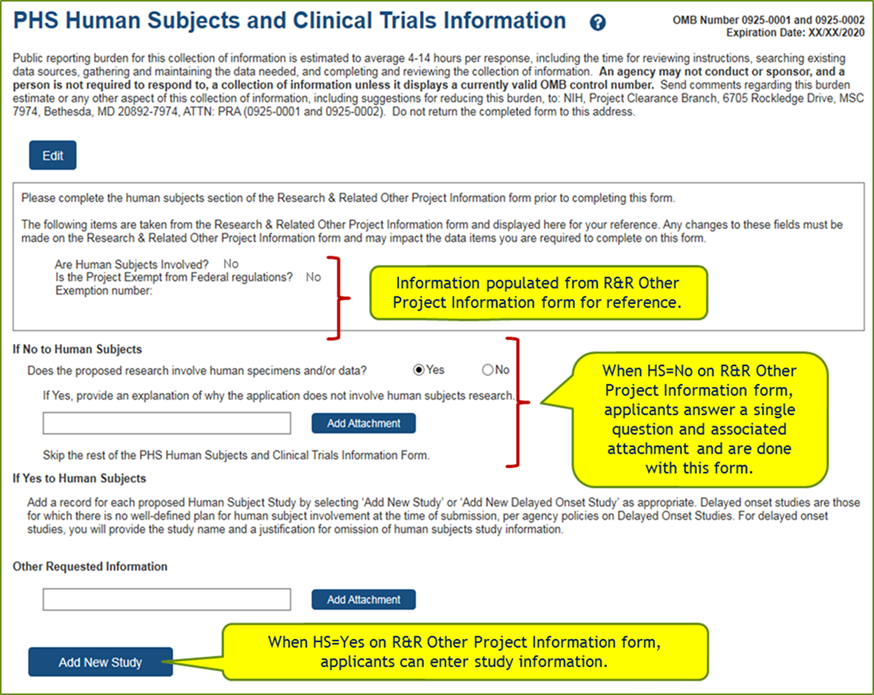
Data
Collection
for
Delayed
Onset
Study

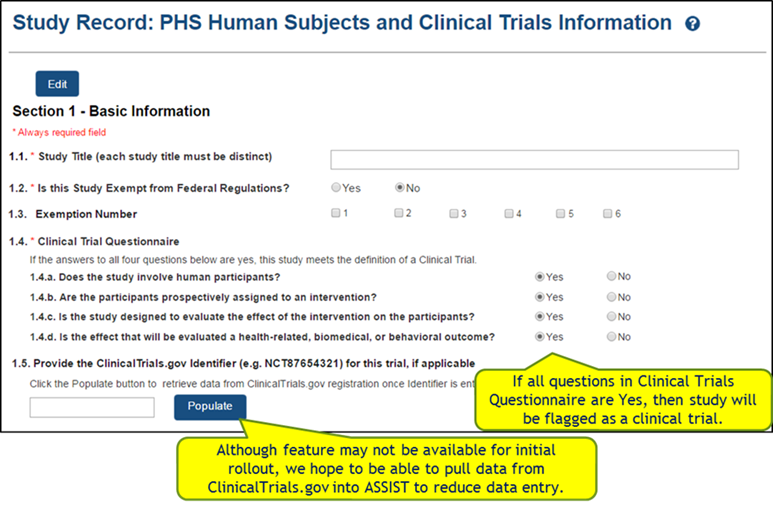
Study
Record:
PHS
Human
Subjects
and
Clinical
Trials
Information
Full
study
records
are
comprised
of 5
sections.
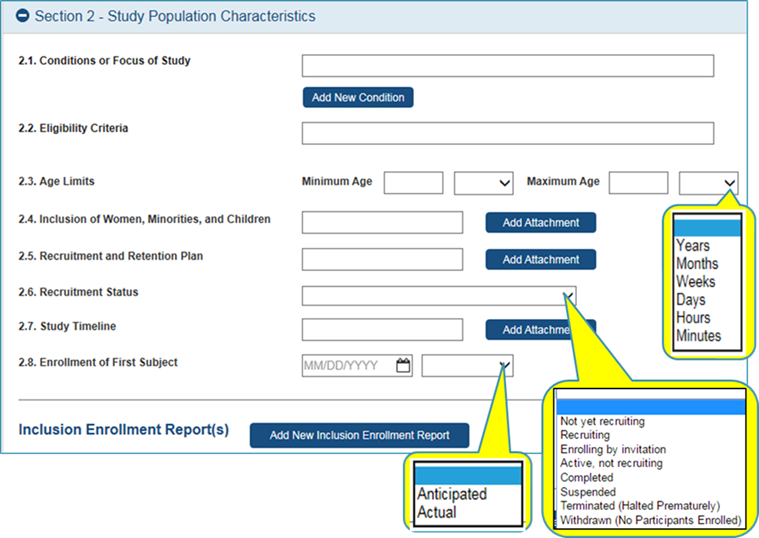
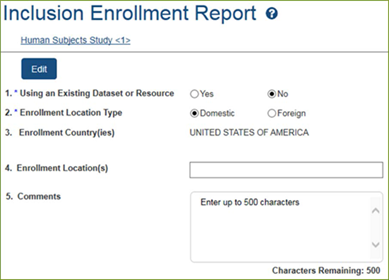
Inclusion Enrollment
Report
Data
Collection will be in Study Record


To unsubscribe from the ROUNDTABLE list, click the following link:
http://listserv.dartmouth.edu/scripts/wa.exe?TICKET=NzM2NzExIEtldmluLk0uR3JhZHlAREFSVE1PVVRILkVEVSBST1VORFRBQkxFIO6yUvUT2sHX&c=SIGNOFF